About Us

About ITC
Since ITC was established in April 1995, we have been manufacturing electro surgical units over 25 years and has been steadily exporting to overseas markets. Since 2008, the company has expanded its export network to Europe with focusing on acquiring certifications, and it is currently being widely sold to Southeast Asia and Europe.
We also has expanded its sales network by producing various aesthetic products used in hospitals, dermatology clinics since 2000.
Recently, with the production of ‘Extracorporeal Shockwave Therapy Device which is a piezo method of innovative technology development, it occupies 40% of the physical therapy device market, taking a large proportion in sales growth.
Our company is continuously developing new products through various R&D support projects and agreements.
We will continue to make steady efforts to become a globally recognized company beyond the Korean medical device market.
Lee Keun Deok
ITC CEO
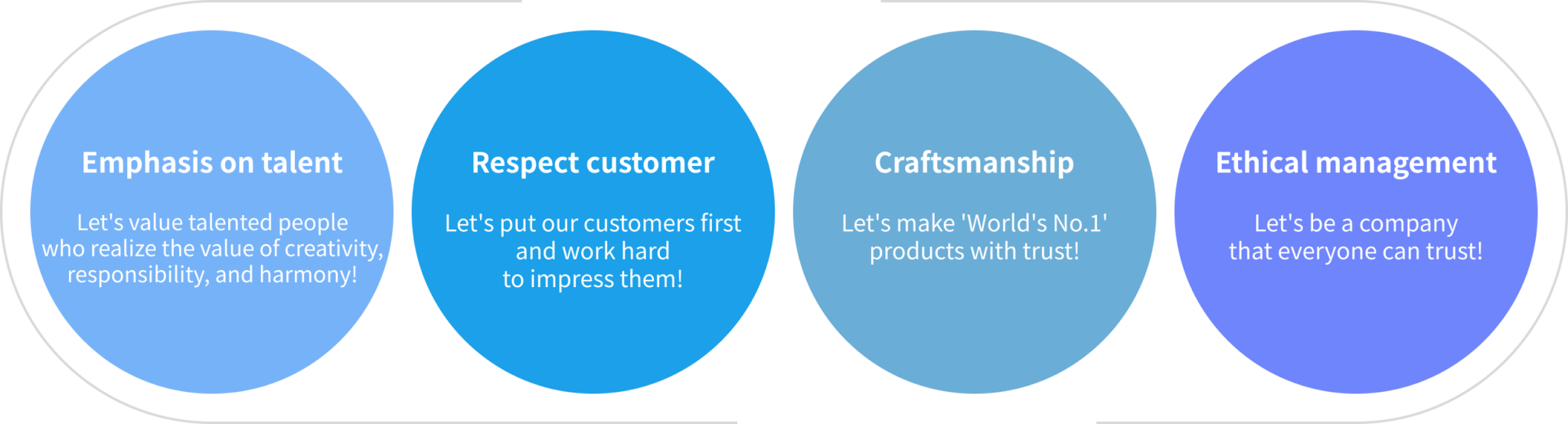
Core Values
ITC History
- April 1995: Establishment of ITC Co., Ltd.
- October 1995: Acquisition of manufacturing license
- August 2005: ITC Co., Ltd. established
- August 2005: Manufacturing License obtained
- June 2007: Selected as a Venture Business
- January 2008: Import GIP Compliance Recognition obtained
- February 2008: Awarded a Citation - Commissioner of the Korea Food and Drug Administration
- April 2008: Established a Corporate Research Institute [Ministry of Science, ITC and Future Planning]
- June 2008: EN ISO9001:2000 / EN ISO 13485:2003 certification renewed
- November 2008: Awarded a Citation - Minister of Knowledge Economy
- June 2009: Awarded a Certificate of Compliance with Medical Device GIP Quality Management Standards
- June 2010: Selected as an INNO-BIZ Technology Innovation - ongoing
- December 2010: Establishment of ITC Central Research Institute
- May 2011: Relocation of ITC's new headquarters [Taplip-dong, Yuseong-gu, Daejeon]
- June 2011: Selected as a Promising Export SME
- October 2013: Selected as an Excellent Design Product - (Extracorporeal Shock Wave Therapy Device RSWT-7000)
- August 2014: Acquired [SZU - EN ISO13485:2012 / ISO13485:2003 & [SZU - CE Certification]: e-Clip
- October 2014: Design Registration - Shock Wave Generator Application No. 2013-0010045
- February 2015: [TUV Rheinland - CE Certification]: FOCUS V-line
- November 2015: [DNV Assurance]: ISO13485:2003 / ENISO 13485:2012 Certification
- March 2016: ITC's new headquarters expanded and relocated[310-7 Techno 2-ro, Yuseong-gu, Daejeon]
- April 2016: Designated as a Youth-Friendly Small and Medium Enterprise - Ministry of Employment and Labor
- June 2016: [TUV-SUD - CE Certified]: V-lineSBotoskin e Focus
- Received Family-Friendly Certification in December 2018
- Selected as a Promising Small and Medium Business by Daejeon City in October 2019
- Selected as a Promising Export SME in July 2020
- Received a Certificate of Commendation from the Minister of SMEs and Startups in December 2020
- January 2021: Selected as a Youth-Friendly Small and Medium Enterprise
- July 2021: Selected as a Daejeon City Star Enterprise
- September 2022: Re-acquired as a Promising Export SME / Ministry of SMEs and Startups
- June 2023: Renewal and Maintenance of EN ISO 13485:2016 Certification / SG Korea
- December 2023: Re-selected as a Daejeon City Star Enterprise (Until December 31, 2025)
